
We will assist you in questions of classification as well as in the preparation of the conformity dossiers and the communication with the notified bodies.Nursing documentation was indiscriminate, and not built into the electronic health record (EHR). The objective purpose, which can be derived from the ingredients in their respective concentration, as well as from the established scientific reports on their main mode of action. included lack of availability of sequential compression device (SCD) pumps, poor patient compliance with use of SCDs, and inadequate nursing documentation.The subjective purpose which the manufacturer assigns to the product.Antiembolic stockings look similar to conventional stockings, but they are much more elastic. Thus, they decrease the risk for blood clots.

They both work by exerting pressure on the veins of the lower legs, promoting blood return to the heart instead of pooling in the legs. In distinguishing products as medical devices from foodstuffs particular attention must be paid to: Antiembolic stockings and SCDs can be used to prevent DVT. s a nurse, you will be asked to apply antiembolic stockings and sequential compression devices, or SCDs, in clients that are at risk for deep vein. Get multiple quotes from competing suppliers and lenders on one platform. Het Superior Canal Dehiscence-syndroom (SCDS), ook wel Superior Semicircular Canal Dehiscence (SSCD) of het derdevenstersyndroom genoemd, is een zeldzame afwijking van het evenwichtsorgaan in het binnenoor.
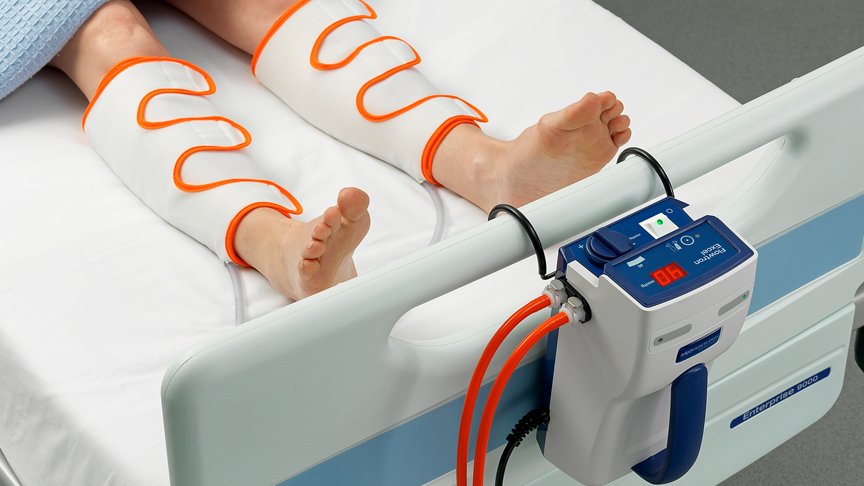

Medical devices are distinguished from medical products mainly by their mode of action, in which the intended main effect of the medical device is not achieved by either a pharmacological, an immunological or a metabolic mode of action, but by other means. Lease/finance, rent, or buy CTC VP500 Sequential Compression Devices (SCDs) starting at 75.00/month. Gumbo Medical gives you quality Sequential Compression Devices at budget-friendly prices. This must be documented in writing as part of the conformity assessment. A Sequential Compression Device (SCD) is a clinically proven. Surplus Inventory Sell Them to MDMaxx Overstock & Liquidation Deals so good, they feel like a mistake Medical Equipment Rental Services> Surplus. The EU rules and regulations call for medical devices to be labeled with a CE label in order to be marketed in Europe and they must comply with the requirements of the medical device law with regard to safety, performance and lack of adverse health effects.


 0 kommentar(er)
0 kommentar(er)
